Emulsify Definition
Emulsification, or to emulsify something, is defined as the mixing of two liquids that usually are unmixable together to form an emulsion. Two liquids can form different types of emulsions depending on which liquid was dispersed in which, with one liquid being the dispersed phase and the other being the external phase, which is added into the dispersed phase. Our ability to emulsify also plays a vital role in the human digestion process and occurs in the small intestine to aid the breakdown of fats.
Different Types of Emulsions
There are three different classes of emulsions: common, microemulsions and nanoemulsions. They are the product of the mixing of immiscible liquids and contain a dispersed phase and an external phase. Their appearance is cloudy due to the scattering of light and has varying degrees of color, dependent on how dilute the emulsion is. Common emulsions are found in items from everyday life, such as milk, mayonnaise and vinaigrettes, and are relatively unstable without the presence of an emulsifier.
They require force to be applied for the emulsion to form, and usually separate back into two phases after some time has passed. Microemulsions and nanoemulsions are defined by droplet size, with both having droplets under 100 nm. The emulsions appear translucent as the droplets are extremely small and do not scatter light as common emulsions do.
Microemulsions are usually spontaneously formed by oil molecules mixed with a stabilizer, such as surfactants, while nanoemulsions can only be formed using specialized machinery.
Emulsification and Digestion
Emulsification plays a vital role in the breakdown of triacylglycerol (TAG) fats in human digestion. When food reaches the stomach, it mixes with acidic secretions to produce chyme. Small amounts of chyme are then propelled by the pyloric sphincter into the duodenum of the small intestine to continue the digestion process. However, fats are hydrophobic, meaning that they repel water, and remain mostly insoluble in the small intestine.
These fats can only be broken down by the enzyme lipase to produce fatty acids and monoglycerides for easier absorption, but the lipase is water soluble so it can only break down the surface of the fat globules. This is where our ability to emulsify becomes vital for digestion.
Emulsification breaks down the fats into smaller manageable droplets and free floating bile salts and phospholipids are recruited and surround each droplet. Bile salts and phospholipids are amphipathic so have both hydrophobic and hydrophilic surfaces and can ensure that large fat globules cannot reform.
Another protein called colipase binds to the surface of these emulsion droplets and helps recruit and anchor lipase to the surface of the droplet. This process of emulsification reduces surface area for the lipase to efficiently digest the fats.
Emulsion Instability
Emulsification mixes two different liquids that do not mix together and makes them intersperse with force. Without the presence of an emulsifier to stabilize the emulsion, the liquids will separate over time. For a successful emulsion to be produced, it must remain combined with no change in droplet size over a significant period of time. There are four different types of instability in emulsions:
- Flocculation: when droplets are attracted to each other and spontaneously form a floc
- Creaming: when droplets rise or sink according to buoyancy
- Coalescence: when droplets merge with each other to form larger droplets
- Ostwald ripening: when smaller droplets decrease in size until they disappear and the free molecules redeposit on larger droplets
Examples of Emulsification
We emulsify liquids all the time in everyday life, from inside the human body, to the food products we consume, as well as products we use. Creams, ointments, and pastes of medicines or health products are all made from stabilized emulsifications. Here are a few examples to illustrate the importance of emulsions in our daily lives:
Emulsions in Food
Many foods we consume regularly are made up of emulsions; for example, to make vinaigrettes for salads, we emulsify oil and vinegar. This emulsion is extremely unstable and without constant mixing will separate very quickly. Mayonnaise and hollandaise sauces are also examples of emulsions; both are oil-in-water emulsions with the addition of egg yolk for stabilization. Butter is another example, and is created when we emulsify water in butterfat.
Emulsification in Milk Production
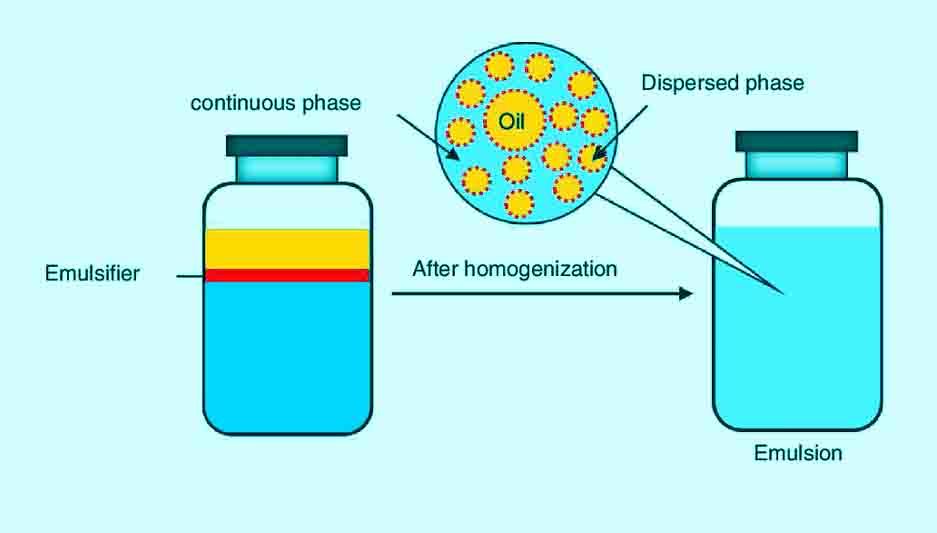
In the old days, milk was purchased and consumed raw with no processing. Nowadays, to increase the shelf life of the milk in supermarkets, it is processed by the emulsification process. Milk is composed of water, protein and fat and if left undisturbed for a period of time, will form two layers.
To prevent this from happening, raw milk is homogenized, meaning the two immiscible liquids are combined into an emulsion. The raw milk is forced at extremely high pressure through tiny holes. This breaks up the fat into tiny particles that are uniformly distributed in the water in milk.
Microemulsions
Microemulsions have particles that are only 400 – 600 nm in diameter. These are now used in many treatments for diseases to deliver vaccines or kill germs. These soybean oil droplets are formed using detergents to stabilize the emulsion and are released into the body. Since they are so small in diameter, they increase surface tension and are strongly attracted to other lipids, causing them to merge.
If these droplets merge with the membranes of bacteria or viruses in large numbers, it causes the membrane to rupture, effectively killing the bacteria/virus. This treatment does not affect most human cells or processes so is relatively specific and targeted. However, this treatment isn’t used intravenously as these microemulsions do affect red blood cells and sperm cells.
Emulsifiers
Emulsifiers or emulsifying agents are vital when we want to successfully emulsify liquids. They are substances that help stabilize an emulsion to allow it to stay in this form for a significant amount of time. Some of the mechanisms they use are as follows:Reducing interfacial tension: Immiscible liquids are separated by their interfacial tension with each other and maintain as small a surface area touching each other as possible. Some emulsifying agents decrease this interfacial tension and allow the liquids to mix seamlessly.
Using repulsion theory: Some emulsifying agents are able to coat droplets of one phase and repel other droplets so they cannot clump and reform larger droplets. This is used in digestion.Increasing viscosity of the medium: Some emulsifiers increase the viscosity of the medium, making it harder for the globules to reform and separate from the dispersed phase.
Some agents used to emulsify liquids are also used to fight fires that originated from the spillage of flammable liquid. They trap the fuel in the water stream, which stops it from burning, effectively stopping the fire from burning and spreading. Some common emulsifiers are as follows:
- Egg Yolks
- Lecithin – this is commonly used in many different types of food
- Mustard
- Sodium phosphates
- Detergents
- Emulsifying wax
Related Biology Terms
- Emulsion – A solution of two immiscible liquids that have been forcefully mixed together.
- Hydrophobic – Molecule that repels in the presence of water.
- Digestion – The process of breaking down food in the gastrointestinal tract.
- Emulsifier – A substance that helps stabilize an emulsion to keep it from separating over time.
FAQ’s
Emulsification is the process of dispersing one immiscible liquid phase within another, resulting in the formation of a stable emulsion. It involves breaking down the larger liquid droplets into smaller droplets and distributing them uniformly throughout the other liquid phase.
Emulsification occurs through the action of emulsifying agents, also known as emulsifiers. These substances contain both hydrophilic (water-loving) and lipophilic (oil-loving) properties, which allow them to interact with both the water and oil phases. Emulsifiers reduce the interfacial tension between the two phases, enabling the formation and stabilization of the emulsion.
Emulsions can be found in various everyday products. Some common examples include mayonnaise, salad dressings, milk, butter, lotions, creams, and cosmetic products. Emulsions are also widely used in industrial applications, such as in the production of paints, coatings, and food additives.
Emulsification is important for several reasons. It helps combine immiscible liquids, allowing for the creation of stable and uniform mixtures. Emulsions can enhance the sensory properties of foods and beverages, improve the delivery of active ingredients in pharmaceuticals or cosmetics, and provide smooth and consistent textures in various products. Emulsification also plays a role in many industrial processes, facilitating efficient mixing, dispersion, and formulation.
Various methods can be employed for emulsification. These include mechanical agitation, such as stirring or shaking, to break down larger droplets into smaller ones. High-shear devices like blenders, homogenizers, or colloid mills are often used to achieve finer and more stable emulsions. Additionally, techniques such as ultrasound and microfluidization can be employed to create emulsions with specific properties. The selection of the emulsification method depends on the nature of the liquids, desired droplet size, and the intended application.